Providence Healthcare

Providence is a Catholic health-care organization and a leader in rehabilitation, palliative care, long-term care and community programs in Toronto.
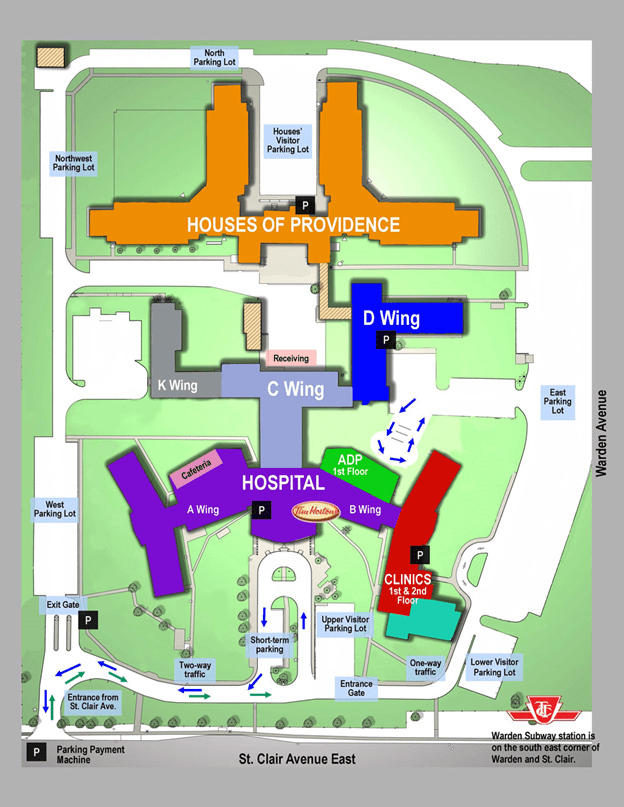
Parking
Paid parking is available on-site. Parking pay stations are located at:
- Hospital main reception
- Clinics entrance
- Palliative Care
- Cardinal Ambrozic Houses of Providence
- The exit gate
On-Site Directions
Click here for Providence Healthcare map pdf
Keeping Our Site Clean
Providence is located in the heart of Scarborough, just steps from some of the city’s most vibrant residential and commercial streets. Whether you’re visiting the site to work, learn, receive care or visit a loved one, it’s important that we treat the site and community with kindness and respect. One way to do this is by keeping the neighbourhood clean. Please be mindful of your garbage and dispose of the following items properly:
- Cigarette butts go in cigarette butt disposals on City of Toronto garbage cans
- Masks and gloves go in garbage bins
- Pop cans go in recycling bins
- Organic waste should be placed in compost bins
Thank you for helping us keep Unity Health and our surrounding neighbourhoods clean. To learn more about how to dispose of various materials, please use the Waste Wizard on the City of Toronto website.

As of April 1, 2021, St. Michael’s Foundation amalgamated with Providence Healthcare Foundation and all receipts will be issued by St. Michael’s Foundation.
We are pleased to let you know that your donation will go directly to the organization and priority that you have designated.
Last updated March 20, 2024